Reactive chemicals play a significant role in various industrial processes, such as manufacturing, chemicals, pharmaceuticals, energy production, and so on. However, their potentially hazardous nature requires careful handling and management to ensure process safety. Reactive chemical services provide valuable expertise in managing the risks associated with these substances, ensuring safe operations, and protecting personnel, the environment, and assets. In this article, we introduce reactive chemical testing to understand chemical reaction hazard.
What tests should be done to assess chemical reaction hazards?
Reactive chemical testing plays a vital role in industrial settings. By utilizing state-of-the-art specialized laboratory equipment and expertise, such as Differential Scanning Calorimeter (DSC), Differential Thermal Analysis (DTA), Thermogravimetric Analysis (TGA), Accelerating Rate Calorimeter (ARC) and adiabatic calorimeter such as Vent Sizing Package (VSP), one can accurately assess the reactivity and thermal hazards and design emergency relief systems associated with various chemical processes.
Differential Scanning Calorimeter (DSC)
DSC is used to measure the heat flow during physical and chemical transitions in a sample as temperature changes. It offers valuable information on exothermic reactions. The applications of DSC in process safety are wide-ranging. It can be used to screen for thermal hazards, assess chemical incompatibilities, characterize materials, and evaluate the thermal stability of products.
DSC operates on the principle of comparing the heat flow to a reference material under controlled temperature conditions. The sample and reference pans are subjected to the same temperature program. The heat flow difference between the sample and reference is continuously monitored, creating a thermal profile that reveals significant events occurring within the sample.
A small quantity of the sample (typically 5 – 20 mg) is loaded into the test cell, which can be constructed from stainless steel, aluminum, or gold-plated cells. For safety studies, sealed high-pressure cells are recommended to prevent evaporative losses. The DSC instrument then ramps the sample, along with an identical reference pan, at a nominal ramp rate of 1 to 10 K·min-1. Higher heat rates can result in lower sensitivity in determining the onset temperature. The DSC measures any exothermic or endothermic activity by assessing the heat flow between the sample and reference pans. The total energy released or absorbed by the sample can be quantified to measure the overall energy of a reaction. Isothermal tests can be performed for studying autocatalytic reactions, while different ramp rates allow for the extraction of formal lump-sum kinetic data.
Typically, a power versus time graph is provided to analyze the thermal reaction. The computer control system interprets this data and provides information on the test graph regarding the onset temperature and energy of the reaction (J·g-1). It should be noted that the obtained onset temperature is not absolute due to factors like the high phi factor and heat losses during testing. For more accurate onset temperature information, the adiabatic calorimeter method is recommended. By analyzing results from multiple tests with different temperature ramp rate, formal kinetic data can be obtained for decomposition reactions. It’s important to note that any decomposition energy exceeding 800 J·g-1 suggests the potential for explosive properties in the material.
From DSC, you may obtain:
- Rection heat in J·g-1.
- Onset T of the endo-exothermic reaction, but with an appropriate safety factor.
- Ideally, reaction kinetic parameters such as activation energy, reaction order, and preexponential factor but need multiple tests with variable scanning rates.
- Induction time of autocatalytic reaction but need expert guidance for testing procedure and data interpretation.
Thermogravimetric Analysis (TGA)
TGA is a powerful analytical technique that involves the controlled heating of a sample and the measurement of its weight changes. This process is conducted in either an inert or oxidizing atmosphere, providing valuable insights into the composition and thermal stability of the sample. The applications of TGA are extensive and include evaluation of thermal stability, examination of oxidative stability, and determination of decomposition kinetics.
The TGA technique involves monitoring the temperature and mass loss of a specimen as it is exposed to a specific environment and heated at a controlled rate. This method allows for a detailed analysis of how the specimen behaves at different temperatures.
The collected data from a thermal reaction is used to create a TGA curve, which illustrates the mass or percentage of the initial mass on the y-axis plotted against temperature or time on the x-axis. By taking the first derivative of the TGA curve, referred to as the DTG curve, inflection points can be identified. These inflection points are helpful for conducting detailed interpretations and differential thermal analysis.
From TGA, you may obtain:
- mass changes vs. temperature
- mass changes vs. time
- reactivity involving air, oxygen, or other reactive gases
- Lumped decomposition or reaction kinetics
Accelerating Rate Calorimeter (ARC)
The Accelerating Rate Calorimeter (ARC) is used to assess the thermal stability of a substance or mixture under adiabatic conditions. This test is commonly conducted to identify the temperature at which an exothermic decomposition begins, as well as to determine the kinetics and magnitude of the resulting runaway reaction.
The ARC instrument can be operated in two modes: the heat-wait-search (HWS) mode and the isothermal age mode. The main principle of this instrument is to maintain the sample under adiabatic conditions once exothermic reaction or self-heating of the sample is detected. This accelerates the reaction rate due to the generated heat.
The test is conducted using a small, metallic sample bomb with a volume of 10 ml. The bomb is filled with 1 to 6 g of the substance being tested. Spherical bombs made from different metals can be used to prevent corrosion or any potential catalytic issues.
The bomb is equipped with a pressure transducer and temperature sensor and placed in the center of an adiabatic enclosure. The HWS method, more commonly used, involves heating the sample step-by-step using a radiant heater. After each heating step, the system waits for thermal equilibration and then searches for any signs of exothermic heat release above the detection threshold (typically set at 0.02 K·min-1). This process is repeated until an exothermic activity is detected, at which point the reaction is actively monitored until completion under adiabatic conditions. The test cell is usually not agitated but possesses a high thermal inertia (phi factor), while having excellent pressure capacity (up to several hundred bar) and high sensitivity.
The onset temperature of an exotherm is identified as the lowest temperature when the detection threshold of the calorimeter is surpassed. By analyzing the raw temperature/pressure/time data, the rates and magnitudes of the reaction can be determined. If the thermal inertia of the cell and the heat capacity of the sample are known, the temperature rise can be converted into the heat of reaction. Additionally, important kinetic parameters like activation energy can be calculated. Despite the high thermal inertia, the calorimetric study allows the temperature data to be corrected using the phi factor, which provides insights into parameters like time to maximum rate. This information, along with supporting data, can be used to estimate the self-accelerating decomposition temperature (SADT) of a sample. It’s worth noting that the onset temperature of activity, if left uncorrected, requires a safety margin of 20°C to 40°C, depending on the specific application.
ARC test typically has a phi factor that falls within the range of 1.5 to 3. This means that mathematical correction of the data is necessary for direct application, or a suitable safety margin should be considered when determining maximum plant exposure temperatures. Since the test vessel is typically not agitated, it may affect the ability to study biphasic systems.
From ARC, you may obtain:
- Adiabatic temperature rise and reaction heat.
- Temperature rise rate and pressure rise rate.
- Vapor-liquid phase equilibrium data – Antoine plot,
- Identify gas generation.
- Identify autocatalytic feature of testing sample.
- Lumpsum reaction kinetic parameters such as activation energy, reaction order, and preexponential factor.
- Onset T of the exothermic reaction, but with an appropriate safety factor.
- Time to maximum rate (TMR) and TD24, i.e., the temperature at which the TMR is 24 hours.
- Temperature of no return (TNR)
- Self-accelerating decomposition temperature (SADT)
- Induction time of autocatalytic reaction but need expert guidance for testing procedure and data interpretation.
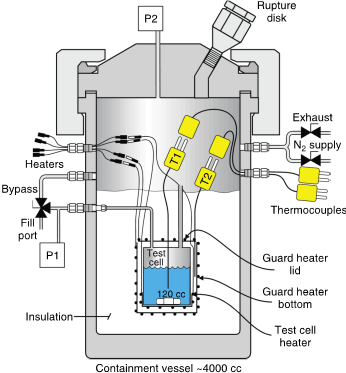
The VSP was developed as part of the DIERS project in the 1980s. The use of VSP in process safety plays a critical role in determining the appropriate sizing of emergency relief systems in industrial processes. The low thermal inertia of this apparatus addresses a common limitation found in other equipment, which is the underestimation of self-heat rate and adiabatic temperature rise. This feature allows laboratory-scale runaway reactions to accurately mimic the severity of those that occur in industrial vessels.
The VSP can be utilized for various purposes, including distinguishing between materials that show homogeneous or non-foamy behavior during emergency relief by measuring the final void fraction in a test cell. It can also determine the impact of viscosity on homogeneous equilibrium flashing flow and measure the necessary parameters for emergency relief device design using graphical or analytical methods. Additionally, it enables direct sizing of emergency relief devices through top- or bottom-vented experiments.
Principle of Operation
VSP is classified as a low phi calorimeter, meaning its thermal inertia factor (Φ-factor) is close to 1, typically ranging from 1.05 to 1.15. To achieve this low thermal inertia factor, a larger volume of reactants, approximately 80 ml, is utilized in a thin-walled metal test cell (can) of 120 ml. However, this design compromises the pressure resistance of the test cell. To compensate for this, the test cell is placed inside an autoclave equipped with a pressure controller. The pressure difference between the test cell contents and the autoclave is kept below a predetermined level by controlling the pressure in the autoclave through nitrogen injections. To maintain adiabatic conditions in the test cell of the calorimeter, a guard heater is employed. This heater ensures that the ambient temperature remains equal to the temperature of the sample. As a result, this calorimeter not only tracks temperature but also pressure. Additionally, the test cell is equipped with a magnetic agitator, enabling operators to conduct reactions that closely resemble conditions found in industrial processes and allowing for experimental simulations of potential runaways.
To gather essential data for analytical ERS sizing methods and experimental validation of proposed ERS designs, different test cells/methods are utilized. These cells acquire information including thermal and pressure data related to runaway reactions, data about the onset/disengagement of two-phase flow (foamy versus nonfoamy), viscosity information to distinguish between turbulent and laminar flow, and additional parameters. The test method is accordingly customized to fit various applications, which can potentially be:
- Closed cell test
- Open cell test for gas generation rate
- Open cell for tempering test
- Blowdown-top vent test for vapor-liquid disengagement characterization
- Blowdown-bottom vent test for viscosity characterization
- And other customized tests.
The rates of temperature and pressure rise, as well as peak reaction temperatures and pressures, are directly obtained from the experimental graphs. The rates of temperature rise can be converted into heat output rates, which can be used to determine the necessary cooling requirements for controlling the reaction at a specific set point. The rates of temperature rise are used in calculating emergency relief systems, while the peak pressures are involved in specifying containment systems. The heat of reaction for the process can be determined by using assumed or measured heat capacity data, as long as the testing cell does not vent before reaching the peak temperature.
From VSPII testing, you may obtain:
- Adiabatic temperature rise and reaction heat.
- Temperature rise rate and pressure rise rate.
- Vapor-liquid phase equilibrium data – Antoine plot,
- Reaction kinetic parameters such as activation energy, reaction order, and preexponential factor.
- Onset T of the exothermic reaction, but with an appropriate safety factor.
- Time to maximum rate (TMR) and TD24, i.e., the temperature at which the TMR is 24 hours
- Temperature of no return (TNR).
- Self-accelerating decomposition temperature (SADT)
- Gas generation rate.
- Tempering capability for an exothermic runaway reaction.
- Identification of flow regime during vessel venting.
- Viscosity features and how it affects flow capacity in relief system.
- Required data for sizing of ERS.
Conclusions:
Analyzing reactive chemical hazards requires specialized expertise, extensive training, and suitable tools. It is essential to have high-quality testing data to conduct accurate assessments of chemical reaction hazards. With state-of-the-art testing equipment and deep expertise, it is feasible to precisely identify and effectively control these hazards within the industry, thereby preventing or reducing risks.