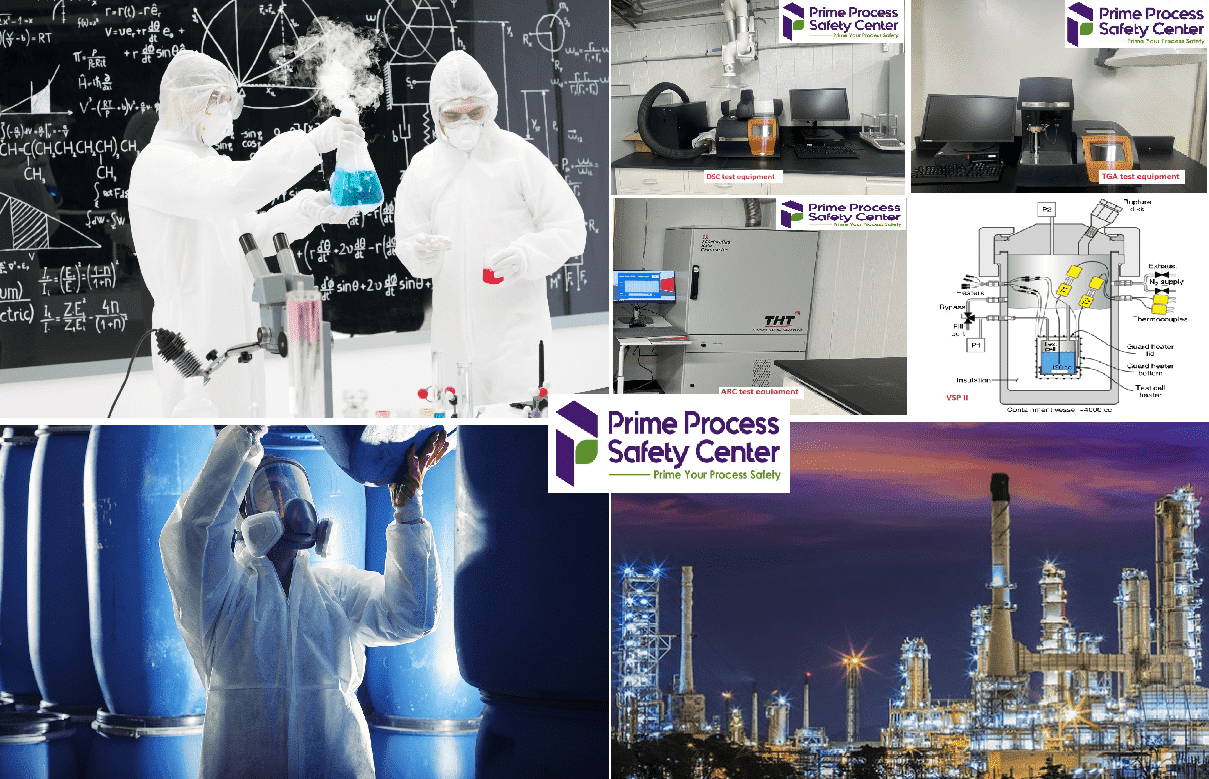
Why the need to test your reactive chemicals?
Reactive chemical testing services play a vital role in process safety for industrial settings. These services involve analyzing and evaluating the properties and behavior of reactive substances to identify potential hazards and ensure safe handling and storage practices. By utilizing state-of-the-art specialized laboratory equipment and expertise, such as Differential Scanning Calorimeter, Differential Thermal Analysis, Thermogravimetric Analysis (Thermogravimetric Analysis), Accelerating Rate Calorimeter (Accelerating Rate Calorimeter), Reaction Calorimeter (mRC or RC-1), and adiabatic calorimeter such as Vent Sizing Package II (Vent Sizing Package II), these services can accurately assess the reactivity and thermal hazards and design emergency relief systems associated with various chemical processes. Ultimately, reactive chemical testing services help to mitigate risks, prevent accidents, and promote a safer working environment within industrial facilities.
What is a Differential Scanning Calorimeter?
A Differential Scanning Calorimeter is an analytical instrument used to measure the heat flows associated with temperature changes in a sample. It provides information on phase transitions, thermal behavior, and energy changes in materials, making it valuable in process safety evaluations.
How does a Differential Scanning Calorimeter work?
In a Differential Scanning Calorimeter, the heat flow difference (or differential heat flow) between a sample and a reference material is measured as they undergo a controlled heating or cooling program. The energy differences indicate phase transitions, reactions, and other thermal events occurring in the sample. Differential Scanning Calorimeter data contributes to understanding the thermal behavior and safety aspects of materials.
What are the applications of Differential Scanning Calorimeter in process safety?
Differential Scanning Calorimeter has diverse applications in process safety assessments, including:
– Determining thermal stability and decomposition behavior of materials under process conditions.
– Identifying potential hazards associated with exothermic reactions, phase changes, or heat flow abnormalities.
– Assessing the compatibility of materials in applications involving temperature variations.
– Evaluating the influence of additives or impurities on the thermal behavior and reactivity of substances.
– Validating stability of active pharmaceutical ingredients (APIs) and other chemical compounds.
What information can be obtained from Differential Scanning Calorimeter measurements?
Differential Scanning Calorimeter measurements provide valuable insight into the thermal properties of materials, including:
– Detection and characterization of phase transitions such as melting points, crystallization, glass transitions, and more.
– Determination of reaction enthalpies and kinetic parameters such as activation energies.
– Evaluation of specific heat capacity and thermal conductivity.
– Quantification of heat flow changes associated with physical or chemical processes.
Can Differential Scanning Calorimeter data be used for process optimization?
While the Differential Scanning Calorimeter primarily focuses on characterizing the thermal behavior and potential hazards of materials, the information obtained from Differential Scanning Calorimeter experiments can be applied to process optimization. By understanding the temperature conditions at which materials undergo phase transitions or reactions, process parameters can be optimized to minimize risks, enhance product quality, and improve overall process efficiency.
What is Differential Thermal Analysis?
Differential Thermal Analysis is a technique used to measure the temperature difference between a sample and a reference material as they are heated or cooled. It provides information on the phase transitions, melting points, reactions, and thermal behavior of substances, making it useful in process safety evaluations.
How does Differential Thermal Analysis work?
In Differential Thermal Analysis, the temperature of the sample and a reference material are simultaneously measured as they undergo a controlled heating or cooling program. A temperature difference between the sample and the reference material indicates thermal events, such as phase transitions or reactions. Differential Thermal Analysis data provides insights into the heat flow associated with these events.
What are the applications of Differential Thermal Analysis in process safety?
Differential Thermal Analysis finds applications in process safety assessments by helping identify potential hazards related to thermal events in materials such as phase transitions, decomposition, or reaction-induced exothermic events. It aids in understanding the thermal behavior of materials and assists in designing safe operating conditions and selecting suitable mitigation measures.
What information can be obtained from Differential Thermal Analysis measurements?
Differential Thermal Analysis measurements can provide valuable information, including:
– Identification of phase transitions, such as melting points, crystallization, and glass transitions, which are important in understanding material behavior and stability.
– Detection of exothermic or endothermic reactions occurring during the temperature ramp, indicating potential hazards.
– Quantification of gas generation.
– Determination of thermal stability and decomposition temperature ranges.
Can Differential Thermal Analysis data be used for process optimization?
Differential Thermal Analysis data primarily focuses on understanding the thermal behavior and potential hazards of materials. However, the information obtained from Differential Thermal Analysis experiments, such as melting points or reaction temperatures, can be crucial in optimizing process conditions. By designing processes to operate below certain critical temperatures or avoiding conditions that induce reactions or phase changes, process safety can be improved.
What is Thermogravimetric Analysis?
Thermogravimetric Analysis is a technique used to measure the change in weight of a sample as it is subjected to a controlled temperature program. It provides information on the composition, thermal stability, and decomposition behavior of materials, making it valuable in process safety evaluations.
How does Thermogravimetric Analysis work?
In Thermogravimetric Analysis, a sample is heated in a controlled atmosphere while its weight change is continuously monitored. As the temperature increases, the sample may lose weight due to decomposition, evaporation, or other chemical reactions. The weight loss or gain is tracked over time, providing valuable information on the material’s thermal behavior.
What are the applications of Thermogravimetric Analysis in process safety?
Thermogravimetric Analysis finds applications in process safety assessments by providing insights into the thermal stability and decomposition behavior of materials. It can help identify potential exothermic reactions, decomposition products, and the temperature range at which hazards can arise. Thermogravimetric Analysis data aids in designing safe operating conditions and selecting appropriate mitigation strategies.
What type of information can be obtained from Thermogravimetric Analysis measurements?
Thermogravimetric Analysis measurements can provide valuable information, including:
– Identification of thermal stability and decomposition temperatures of materials.
– Quantification of weight loss or gain as a function of temperature or time.
– Assessment of the potential for hazardous gas evolution during heating.
Can Thermogravimetric Analysis data be used for process optimization?
Thermogravimetric Analysis data is primarily used for evaluating the thermal hazards of materials. However, the information obtained from Thermogravimetric Analysis experiments, such as decomposition rates or temperature ranges, can be used to optimize process conditions to prevent undesired decomposition or reaction events. It helps in designing reactions or selecting materials that can withstand desired operating conditions while minimizing potential safety risks.
What is an Accelerating Rate Calorimeter?
An Accelerating Rate Calorimeter is a specialized instrument used to evaluate thermal hazards and determine the temperature and pressure rise rate of reactions, mixtures, and materials. It is extensively used in process safety assessments to identify hazards related to exothermic reactions and reactive materials.
How does an Accelerating Rate Calorimeter work?
An Accelerating Rate Calorimeter typically consists of a small 10 ml sample bomb that holds the reaction or mixture being analyzed. The instrument monitors the temperature of the sample while a controlled heat source is applied, allowing for the measurement and assessment of the heat release rate. The Accelerating Rate Calorimeter provides valuable data on the potential dangers of a reaction, including onset temperature, self-heating rates, heat of reaction, and time to maximum rate of heat release.
Why is an Accelerating Rate Calorimeter important in process safety?
Accelerating Rate Calorimeter plays a critical role in process safety by providing insights into the thermal behavior and hazards of reactions. It helps identify potential runaway reactions and thermal explosions, aids in designing safe reaction conditions, and assists in evaluating and selecting appropriate preventive measures for process safety.
What are the advantages of using an Accelerating Rate Calorimeter?
Using an Accelerating Rate Calorimeter offers several advantages, such as:
– Early identification of potential thermal hazards during a reaction or with reactive materials.
– Quantitative measurement of heat release rates, allowing for the accurate assessment of reaction hazards.
– Generating valuable data for process safety assessments and enabling the development of effective safety protocols.
– Assisting in the selection and evaluation of preventative measures, such as temperature control, venting systems, or the use of specialized equipment and materials.
Can an Accelerating Rate Calorimeter be used for scale-up considerations?
While an Accelerating Rate Calorimeter primarily focuses on evaluating small-scale thermal hazards, the data obtained from its measurements can be informative for scale-up considerations. By providing insights into the heat release rate and potential thermal hazards, an Accelerating Rate Calorimeter can help guide the design and safety considerations when scaling up a process. However, additional measurements and analyses would usually be required to ensure safety at larger production scales
What is reaction calorimetry?
Reaction calorimetry is a technique used to measure the heat generated or consumed during a chemical reaction. It provides valuable information about the thermal behavior and safety hazards of reactions, helping in the optimization of process conditions and the assessment of potential risks.
Why is reaction calorimetry important in process safety?
Reaction calorimetry plays a crucial role in process safety by providing insights into the thermal behavior of reactions. It helps to identify and mitigate potential hazards associated with exothermic reactions, such as runaway reactions or thermal explosions. By understanding the heat release and heat transfer during reactions, process engineers can design safer and more efficient processes.
How is reaction calorimetry performed?
Reaction calorimetry involves conducting experiments in specialized calorimeters designed to measure heat effects. Typically, reactants are mixed in a calorimeter, and the heat generated or consumed is measured through temperature changes. Data obtained from these experiments can be used to calculate heat transfer coefficients, heat capacity, reaction kinetics, and other parameters relevant to process safety.
What are the benefits of using reaction calorimetry in process safety studies?
Using reaction calorimetry in process safety studies provides several advantages. It allows for the early identification of hazardous reactions, facilitates the optimization of reaction conditions to enhance safety, and provides data necessary for the design of safe operating processes.
Can reaction calorimetry be used for scale-up purposes?
Yes, reaction calorimetry data can be used for scale-up purposes. By understanding the heat generation or consumption behavior of reactions at a small scale, it is possible to predict and control the thermal behavior when scaling up to larger production volumes. This helps in ensuring the safety and efficiency of the process at different scales, minimizing the risks associated with large-scale reaction.
What is a Vent Sizing Package apparatus?
The Vent Sizing Package was introduced in 1985 by DIERS for characterizing runaway reactions. The advantages of the Vent Sizing Package include a lightweight test cell and a resulting small Phi factor (low thermal inertia), as well as adiabatic pressure tracking and heat-wait-search capabilities. Moreover, the adiabatic operation permits direct application of temperature and pressure data in large-scale vessels.
How does the vent sizing package apparatus work?
The Vent Sizing Package can be considered as a bench-scale chemical reactor housed within a protective containment vessel. It allows for the addition or withdrawal of liquid or gaseous reactants at any point during an experiment. Tests can be conducted in true adiabatic mode, with capability of external heating or cooling.
Why is vent sizing important in process industries?
Proper vent sizing ensures the safety and integrity of vessels and process equipment. It prevents dangerous pressure build-up that could lead to equipment failure or other hazardous incidents. Adhering to vent sizing requirements is essential for mitigating risks and maintaining the overall safety of the operation.
Are there any specific regulations or codes governing vent sizing?
Yes, there are various industry codes and standards that provide guidelines for vent sizing, such as API 520 and API 521 in the petrochemical industry. These standards ensure compliance with safety regulations and help engineers design reliable pressure relief systems that meet industry requirements.
Can the vent sizing package apparatus be used for different industries?
Yes, the vent sizing package apparatus can be utilized in a wide range of industries, including petrochemical, pharmaceutical, food processing, and energy sectors. It is crucial for any industry that deals with processes involving pressure vessels to ensure proper vent sizing and pressure relief for safe operations.
What is Self-Accelerating Decomposition Temperature?
Self-Accelerating Decomposition Temperature refers to the lowest temperature at which a self-reactive substance or an organic peroxide can undergo self-accelerating exothermic decomposition. This temperature is crucial for ensuring the safe storage, handling, and transportation of potentially hazardous materials.
Why are Self-Accelerating Decomposition Temperature tests important?
Self-Accelerating Decomposition Temperature tests are vital for determining the safe storage and transportation conditions of reactive chemicals. They help prevent thermal runaway reactions, which can lead to fires, explosions, or toxic releases, thus ensuring workplace and environmental safety.
- How is Self-Accelerating Decomposition Temperature determined?
Self-Accelerating Decomposition Temperature is determined by gradually heating a sample of the material in a controlled environment, such as an Accelerating Rate Calorimeter, and monitoring for exothermic reactions. The lowest temperature at which a self-accelerating reaction is detected within a specified timeframe is the Self-Accelerating Decomposition Temperature.
- What types of materials require Self-Accelerating Decomposition Temperature testing?
Self-Accelerating Decomposition Temperature testing is typically required for self-reactive substances and organic peroxides, especially those that are prone to undergo exothermic decomposition. These materials are often found in the chemical manufacturing, pharmaceutical, and transportation industries.
- How does Self-Accelerating Decomposition Temperature impact safety procedures?
The Self-Accelerating Decomposition Temperature data informs the development of safety procedures and guidelines for the handling and storage of reactive chemicals. It helps in setting temperature controls, packaging requirements, and emergency response plans to mitigate the risk of accidental thermal runaway and ensure regulatory compliance.
What is reactive chemical testing?
Reactive chemical testing involves analyzing chemicals to determine their reactivity, stability, and potential hazards when they interact with other substances or conditions.
Why is reactive chemical testing important?
It helps identify and mitigate risks associated with chemical reactions, preventing accidents such as fires, explosions, and toxic releases.
What industries commonly require reactive chemical testing?
Industries such as chemical manufacturing, pharmaceuticals, petrochemicals, and laboratories often require reactive chemical testing.
What are the key objectives of reactive chemical testing?
To identify potential reactive hazards, understand reaction mechanisms, and determine safe handling, storage, and disposal procedures.
What types of reactions are typically investigated in reactive chemical testing?
Reactions involving oxidation, reduction, polymerization, decomposition, and interactions with water or other chemicals.
What are some common methods used in reactive chemical testing?
Methods include Differential Scanning Calorimeter, Accelerating Rate Calorimeter, thermal stability tests, and chemical compatibility tests.
What is a Differential Scanning Calorimeter?
Differential Scanning Calorimeter measures the heat flow associated with chemical reactions as a function of temperature, helping identify exothermic and endothermic reactions.
What is an adiabatic calorimeter?
An adiabatic calorimeter, such as an Accelerating Rate Calorimeter measures the heat and pressure generated by a reaction under adiabatic conditions, providing data on the reaction’s runaway potential.
What is the importance of identifying exothermic reactions?
Exothermic reactions release heat, which can lead to thermal runaway, fires, or explosions if not properly managed.
What is chemical compatibility testing?
Testing to determine how different chemicals interact when mixed, to avoid dangerous reactions that could occur during storage or processing.
What is a runaway reaction?
A runaway reaction is an uncontrolled reaction that accelerates rapidly, often resulting in a significant release of heat and pressure.
How is thermal stability assessed in reactive chemical testing?
By subjecting a chemical to controlled heating and observing its decomposition or reaction behavior, typically using a Differential Scanning Calorimeter or thermogravimetric analysis.
What is the role of the Material Safety Data Sheet (MSDS) in reactive chemical testing?
MSDS provides essential information on the chemical’s properties, hazards, and handling precautions, serving as a reference for reactive testing.
What are some common signs of a potentially reactive chemical?
Signs include rapid temperature rise, gas evolution, color change, and pressure buildup when the chemical is exposed to certain conditions.
What is the importance of reaction kinetics in reactive chemical testing?
Understanding reaction kinetics helps predict the rate of reaction and the conditions under which a reaction may become hazardous.
How do you determine the safe storage conditions for reactive chemicals?
Through reactive chemical testing, which identifies temperature limits, compatible storage materials, and necessary safety measures.
What is the significance of pressure relief systems in managing reactive chemicals?
Pressure relief systems help mitigate the risk of overpressure and explosions by safely venting excess pressure generated during a reaction.
What is the role of a chemical process safety engineer in reactive chemical testing?
To design and oversee testing protocols, analyze data, and develop safety strategies for handling reactive chemicals.
How can reactive chemical testing prevent industrial accidents?
By identifying hazardous reactions before they occur, allowing for the implementation of controls and safety measures to prevent accidents.
What are some common instruments used in reactive chemical testing?
Instruments include Differential Scanning Calorimeters, Accelerating Rate Calorimeter, thermogravimetric analyzers, and reaction calorimeters.
How do you handle unexpected results in reactive chemical testing?
By reviewing test procedures, re-evaluating the chemical properties, and conducting further tests to confirm and understand the findings.
What is a chemical reactivity hazard analysis?
A systematic evaluation of chemical reactivity data to identify and control hazards associated with chemical processes and storage.
What is the importance of scale-up studies in reactive chemical testing?
Scale-up studies ensure that reactions that are safe at laboratory scale remain safe when implemented at industrial scale, identifying potential hazards unique to larger volumes.
How do you assess the compatibility of process materials with reactive chemicals?
By conducting compatibility tests to determine if process materials (e.g., containers, piping) react adversely with the chemicals used.
What is a thermal runaway reaction?
A thermal runaway reaction occurs when the heat generated by a reaction causes an increase in reaction rate, leading to a self-accelerating, uncontrolled reaction.
What are the regulatory requirements for reactive chemical testing?
Requirements vary by region but generally include compliance with OSHA standards, EPA regulations, and industry-specific guidelines for chemical safety.
How do you ensure the accuracy of reactive chemical testing?
By calibrating instruments regularly, following standardized testing protocols, and conducting repeat tests to verify results.
What are some examples of hazardous reactive chemical incidents?
Incidents include the T2 Laboratories explosion (2007) and the BASF explosion (1947), both of which underscore the importance of understanding chemical reactivity.
What is the role of emergency response planning in reactive chemical safety?
Emergency response planning prepares for potential accidents involving reactive chemicals, ensuring quick and effective response to minimize harm.
How do you communicate reactive chemical hazards to employees?
Through safety training, clear labeling, providing MSDS, and conducting regular safety meetings to ensure employees are aware of the hazards and proper handling procedures.
What Services does Prime Process Safety Center offer
Prime Process Safety Center offers the following Reactive Chemical Consulting Services; Chemical Reaction Hazard Assessment, Calorimetric Studies, Chemical Compatibility Studies, Chemical Instability, Chemical Kinetics Evaluation, Process Dynamic Simulation, Self-Heating Evaluation and Analysis, Emergency Relief System Design, DIERS Technology for Two-Phase Relief System Design and Emergency Relief Effluent Handling System Design
Moreover, Prime Process Safety Center offers the following reactive chemical testing services Differential Scanning Calorimeter (Differential Scanning Calorimeter), Differential Thermal Analysis (Differential Thermal Analysis), Thermogravimetric Analysis (Thermogravimetric Analysis), Accelerating Rate Calorimeter (Accelerating Rate Calorimeter) and Vent Sizing Package (Vent Sizing Package)

