We also offer
What is Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) measures heat flow during physical and chemical transitions in a sample as temperature changes. It provides valuable data on melting, crystallization, phase transitions, and chemical reactions.
In process safety, DSC plays a key role in screening for thermal hazards, assessing chemical incompatibilities, evaluating thermal stability, and characterizing materials.
Applicable Standard: ASTM E537 – Standard Test Method for the Thermal Stability of Chemicals by Differential Scanning Calorimetry
Principle of Operation
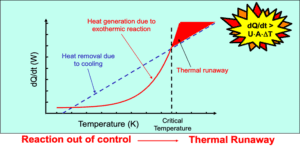
Differential Scanning Calorimetry (DSC) compares the heat flow of a test sample against a reference material under controlled temperature conditions. Both pans follow the same heating program, and the instrument continuously monitors heat flow differences. The resulting thermal profile reveals events such as melting points, exothermic reactions, or decomposition behavior.
Test Method
-
A small sample (5–20 mg) is placed in a test cell, typically stainless steel, aluminum, or gold-plated.
-
For safety studies, sealed high-pressure cells are recommended to prevent evaporative losses.
-
The DSC ramps the sample and reference pan at a rate of 1–20 K·min⁻¹. Higher heating rates may reduce sensitivity in detecting onset temperatures.
-
The instrument measures exothermic and endothermic reactions, quantifies total heat release or absorption, and provides data on reaction energetics.
-
Isothermal tests allow study of autocatalytic reactions, while varying ramp rates can yield kinetic data.
Data Interpretation
DSC results are typically displayed as a power versus time graph. The system calculates reaction onset temperatures and energy release (J·g⁻¹).
-
Onset temperatures require applying safety factors, especially at high heating rates (>5 K·min⁻¹).
-
Adiabatic calorimetry provides more accurate onset temperatures.
-
Decomposition energy values can be used directly.
-
Comparing air and nitrogen atmospheres distinguishes oxidative reactions from pure decomposition.
-
Multiple ramp rate tests allow extraction of kinetic parameters.
Any decomposition energy above 800 J·g⁻¹ may indicate explosive potential.
Key results from DSC include:
-
Reaction heat (J·g⁻¹).
-
Onset temperature of endo/exothermic events, with safety factors applied.
-
Kinetic parameters (activation energy, reaction order, pre-exponential factor).
-
Induction time for autocatalytic reactions (requires expert guidance).
Why Perform Differential Scanning Calorimetry (DSC) Testing
Differential Scanning Calorimetry is crucial in process safety for:
-
Identifying thermal behavior of materials.
-
Detecting hazards linked to phase transitions or abnormal heat flow.
-
Evaluating stability and compatibility of chemicals.
-
Supporting safer process design and optimized operating conditions.
-
Preventing incidents by highlighting risks of runaway reactions or decomposition.
DSC data strengthens safety planning, improves process efficiency, and ensures product quality.
Why Choose Prime Process Safety Center
-
Expertise: Skilled professionals experienced in DSC testing and data interpretation.
-
Advanced equipment: State-of-the-art DSC instruments for precise, sensitive measurements.
-
Strict protocols: Rigorous quality control for reliable and consistent results.
- Accreditation: Testing performed in our ISO/IEC 17025:2017 accredited laboratory.
-
Valuable insights: In-depth analysis and tailored recommendations for your applications or research.
FAQ
1. What is a Differential Scanning Calorimeter (DSC)?
A Differential Scanning Calorimeter (DSC) is an analytical instrument used to measure the heat flows associated with temperature changes in a sample. It provides information on phase transitions, thermal behavior, and energy changes in materials, making it valuable in process safety evaluations.
2. How does DSC work?
In DSC, the heat flow difference (or differential heat flow) between a sample and a reference material is measured as they undergo a controlled heating or cooling program. The energy differences indicate phase transitions, reactions, and other thermal events occurring in the sample. DSC data contributes to understanding the thermal behavior and safety aspects of materials.
3. What are the applications of DSC in process safety?
DSC has diverse applications in process safety assessments, including:
- Determining thermal stability and decomposition behavior of materials under process conditions.
- Identifying potential hazards associated with exothermic reactions, phase changes, or heat flow abnormalities.
- Assessing the compatibility of materials in applications involving temperature variations.
- Evaluating the influence of additives or impurities on the thermal behavior and reactivity of substances.
- Validating stability of active pharmaceutical ingredients (APIs) and other chemical compounds.
4. What information can be obtained from DSC measurements?
DSC measurements provide valuable insight into the thermal properties of materials, including:
- Detection and characterization of phase transitions such as melting points, crystallization, glass transitions, and more.
- Determination of reaction enthalpies and kinetic parameters such as activation energies.
- Evaluation of specific heat capacity and thermal conductivity.
- Quantification of heat flow changes associated with physical or chemical processes.
5. Can DSC data be used for process optimization?
While DSC primarily focuses on characterizing the thermal behavior and potential hazards of materials, the information obtained from DSC experiments can be applied to process optimization. By understanding the temperature conditions at which materials undergo phase transitions or reactions, process parameters can be optimized to minimize risks, enhance product quality, and improve overall process efficiency.