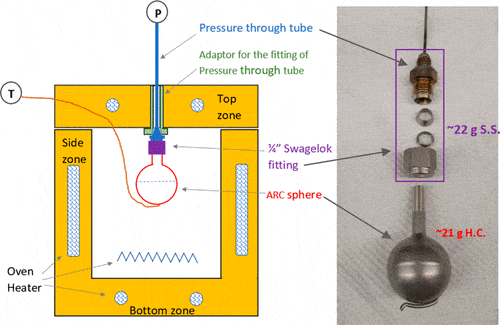
We also offer
Self-Heating Evaluation and Analysis
Self-heating reactions present a significant challenge in process safety, requiring thorough evaluation and analysis to prevent potential hazards. These reactions, which generate heat as they progress, can escalate into uncontrolled fires, explosions, or thermal runaway if not appropriately managed. Incidents of self-heating in processing equipment like dryers, dust collectors, silos, and FIBCs (super sacks) have resulted in numerous fires and explosions across various industries.
To effectively assess potential risks and implement appropriate safety measures, it is crucial to understand the underlying mechanisms and kinetics that govern self-heating reactions. Two widely recognized theories—Semenov theory and Frank-Kamenetskii theory—offer different approaches for analyzing and studying self-heating reactions.
Semenov Theory
Semenov theory, developed by Russian scientist Nikolay Semenov, provides a detailed understanding of self-heating in gas and low-viscosity liquid systems. The theory is based on three assumptions that restrict its applicability:
-
Spatially uniform temperature within the reacting body.
This assumption implies that the material is either well-stirred (as in liquid or gas systems) or that resistance to heat flow within the body is much lower than within the container or boundary. In the latter case, a temperature discontinuity appears at the boundary. This assumption is a good approximation for deliberately stirred fluids. -
The heat-generation reaction can be simplified to a single chemical reaction.
This approximation is often valid when a “lumped” or empirically determined rate law has been independently measured. It remains accurate in many systems that do not involve single-step reactions. -
Both the heat of reaction and activation energy are sufficiently high to facilitate self-heating and ignition behavior.
These assumptions are based on the understanding that without reaction heat, self-heating would not occur, and without activation energy (the rate at which the reaction accelerates with temperature), ignition would not take place.
Frank-Kamenetskii Theory
While Semenov theory focuses on self-heating in well-stirred systems, the Frank-Kamenetskii theory, proposed by Soviet scientist Zeldovich Frank-Kamenetskii, emphasizes thermal conductivity and heat transfer within self-heating materials.
This theory provides a fundamental understanding of the thermal processes involved in exothermic reactions, particularly when heat transfer controls the reaction rate. As a result, it can be applied to self-heating systems that are solid or highly viscous, since it accounts for temperature distribution inside the materials. Thus, only assumptions 2 and 3 of Semenov theory apply to the Frank-Kamenetskii theory.
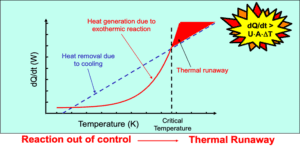
Both theories revolve around key parameters such as heat generation (related to reaction kinetics and heat of reaction) and heat loss (linked to factors like heat-transfer area, coefficient, and ambient temperature). If the rate of heat generation exceeds the rate of heat removal, self-heating may escalate into a runaway or ignition event.
The critical condition, representing the boundary between stable and runaway behavior, plays a pivotal role in self-heating evaluation. It depends on factors such as the surrounding temperature, the size and shape of the material, and its internal temperature.
Critical Condition Parameters
Critical Ambient Temperature (CAT):
The CAT is conceptually similar to the Self-Accelerating Decomposition Temperature (SADT). It is the lowest surrounding temperature at which thermal runaway or ignition could occur for a given size and shape of self-heating material—or conversely, the highest surrounding temperature at which it would not occur.
Critical Size and/or Shape:
In constant-temperature environments, the critical size or diameter determines the threshold at which thermal runaway or ignition occurs. It represents the maximum size where ignition would not occur, or the minimum size where it would occur.
Critical Stacking Temperature (CST):
CST refers to the maximum temperature at which a material stack can be safely maintained without risking thermal runaway or ignition. It depends on material properties, thermal characteristics, degradation potential, and exothermic reactivity. Both the chemical reactions occurring within the material and the heat-transfer mechanisms must be considered when determining CST.
Why Perform Self-Heating Evaluation and Analysis
By incorporating self-heating evaluation and analysis into process safety programs, industries can significantly reduce the risk of fires, explosions, and thermal runaway. Proactive identification and mitigation of self-heating hazards protect personnel and assets while ensuring compliance with safety regulations.
This approach leads to:
-
A safer working environment
-
Improved operational efficiency
-
Enhanced regulatory compliance
-
Preservation of company reputation
Self-heating evaluation is a vital component of a comprehensive process safety strategy, ensuring long-term safety and reliability.
Why Choose Prime Process Safety Center
Prime Process Safety Center is a leading provider of process safety consulting and testing services, offering expertise in self-heating evaluation and analysis to help industries navigate the challenges of exothermic reactions safely. Our work is supported by our ISO/IEC 17025:2017 accredited laboratory, ensuring accuracy and confidence in every result.
By prioritizing self-heating evaluation and analysis, we help industries achieve operational excellence, safeguard their reputation, and promote a culture of safety. Through its deep understanding of self-heating phenomena, Prime Process Safety Center empowers organizations to ensure safety, efficiency, and environmental responsibility—paving the way for a safer and more sustainable future.
FAQ
1. What is self-heating?
Self-heating refers to the process by which a material, typically organic or inorganic solids, undergoes an exothermic reaction and generates heat internally. This heat generation can lead to an increase in temperature, potentially causing a fire or explosion.
2. Why is self-heating evaluation important?
Self-heating evaluation is crucial in process safety because it helps identify materials that may have the potential for self-heating and assess their potential ignition risks. It allows industries to develop appropriate control measures to prevent incidents related to self-heating.
3. How is self-heating evaluation conducted?
Self-heating evaluation involves analyzing the properties and characteristics of materials such as their chemical composition, environmental conditions, heat capacity, and reaction kinetics. Usually, oven-basket test with varying basket sizes is used to deduce the lump-sum kinetic parameters and physical properties. This information is used to evaluate the likelihood and severity of self-heating.
4. Who should perform self-heating evaluations in industries?
Self-heating evaluations are typically conducted by process safety experts, such as chemical engineers, industrial hygienists, or safety consultants who are knowledgeable in the properties and behaviors of hazardous materials.
5. How frequently should self-heating evaluations be carried out?
The frequency of self-heating evaluations depends on various factors, including the type of materials being handled, their storage and handling conditions, as well as any changes in the process or material that may affect self-heating characteristics. It is generally recommended to conduct evaluations regularly or when changes occur.
6. What control measures can be implemented to mitigate self-heating risks?
Control measures for self-heating risks may include maintaining adequate ventilation, controlling temperature and humidity, implementing appropriate storage and handling practices, conducting regular inspections and preventive maintenance, and implementing a robust monitoring system such as carbon monoxide gas monitoring.
7. What are some common materials prone to self-heating?
Common materials prone to self-heating include oily rags, certain chemical compounds containing unsaturated bond or peroxides, coal, biomass, vegetable oils, and greases. These materials can undergo exothermic reactions and generate heat if not managed properly.
8. How can self-heating evaluations help in preventing incidents?
By identifying materials prone to self-heating and assessing their risks, industries can implement preventive measures such as proper storage, ventilation, and monitoring, reducing the likelihood of incidents caused by self-heating.
9. Can self-heating be completely eliminated?
Complete elimination of self-heating may not always be possible due to inherent properties of certain materials or operational requirements. However, through diligent evaluation, control measures, and monitoring, the likelihood of self-heating can be significantly reduced.
10. How can self-heating evaluation contribute to regulatory compliance?
Self-heating evaluations ensure compliance with safety regulations by identifying and assessing self-heating risks. It helps industries adhere to standards and guidelines set by regulatory bodies that mandate the safe handling and storage of materials prone to self-heating.