Introduction
Reactive chemical testing plays a vital role in industrial safety and process safety management (PSM), especially in sectors where exothermic reactions, thermal decomposition, or unstable compounds are present. The main goal is to identify, characterize, and quantify the potential for hazardous energy release—such as fires, explosions, or toxic gas evolution—during both normal operations and abnormal conditions.
By understanding the reactivity hazards of individual chemicals and their mixtures, safety professionals and engineers can implement appropriate process controls, establish safe operating limits, and design effective emergency response strategies. Reactive chemical testing supports both operational safety and regulatory compliance, making it essential for high-risk industries including pharmaceuticals, chemicals, and specialty manufacturing.
Nature and Scope of Reactive Hazards
Reactive hazards stem from materials or combinations that can undergo uncontrolled chemical reactions. These include:
-
Self-reactive substances (e.g., organic peroxides)
-
Incompatible chemicals (e.g., acids with bases, oxidizers with fuels)
-
Thermally unstable compounds (e.g., nitrates, azides)
-
Runaway reactions in batch reactors or during storage
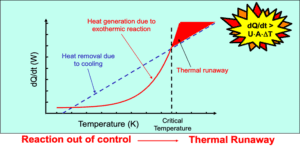
If left unchecked, these reactions can generate excessive heat or pressure, leading to equipment rupture and the release of flammable, toxic, or corrosive byproducts. For example, notable industrial disasters—such as the T2 Laboratories explosion in 2007 and the Seveso disaster in 1976—clearly illustrate the devastating consequences of neglecting proper reactive hazard assessment.
Objectives of Reactive Chemical Testing
The purpose of reactive chemical testing is to:
-
Identify thermal decomposition and instability hazards
-
Evaluate potential runaway reaction scenarios
-
Determine chemical compatibility with other materials
-
Quantify energy release, pressure rise rates, and onset temperatures
-
Support the design of emergency relief systems using DIERS methodology
-
Guide scale-up decisions during process development
These objectives help ensure safety during every phase—from R&D through full-scale production.
Common Testing Methods
Understanding which testing methods to use is key to characterizing chemical reactivity hazards effectively:
Differential Scanning Calorimetry (DSC)
Used to identify onset temperatures, melting points, or decomposition behavior. Ideal for preliminary screening.
Accelerating Rate Calorimetry (ARC)
Provides insight into adiabatic temperature rise, self-heating behavior, and time-to-maximum rate—critical for assessing thermal runaway potential.
Vent Sizing Package 2 (VSP2) / Phi-TEC II
Used under realistic process conditions to determine pressure generation and support emergency relief vent sizing.
Thermogravimetric Analysis (TGA)
Measures mass changes during controlled heating—helpful for detecting thermal degradation or moisture content.
Reactive Compatibility Testing
Includes mixing calorimetry, Dewar tests, and others to detect violent or unexpected chemical interactions.
Interpretation of Results
Once data is collected from reactive chemical testing, it must be interpreted in the context of process design. Key parameters include:
-
Onset temperature
-
Heat of reaction (ΔH)
-
Adiabatic temperature rise
-
Gas or vapor evolution
-
Pressure-time behavior
These values inform hazard identification techniques such as HAZOP, LOPA, or SIL studies, and are critical for safe process scale-up and equipment design.
Relevance in the Pharmaceutical Industry
Reactive chemical testing in pharmaceutical manufacturing is especially important due to the nature of active pharmaceutical ingredients (APIs), intermediates, and novel compounds. Common high-risk operations include:
-
Drying thermally unstable intermediates
-
Reactions involving strong oxidizers or reducing agents
-
Scale-up of new chemical entities (NCEs)
Regulatory authorities like the FDA and EMA expect thorough safety data as part of the development pipeline. In addition, Good Manufacturing Practice (GMP) guidelines require safe handling, containment, and segregation of reactive materials.
Regulatory and Best Practice Guidance
Reactive chemical testing supports compliance with numerous safety standards and regulations, including:
-
OSHA PSM (29 CFR 1910.119)
-
EPA RMP (40 CFR Part 68)
-
NFPA 69 – Standard on Explosion Prevention Systems
-
NFPA 704 – Hazard identification system
-
CCPS Guidelines – Best practices for chemical reactivity hazard management
-
ASTM E1981 / E1445 – Reactivity testing standards
It also helps meet international transport, storage, and handling regulations for reactive or hazardous materials.
Conclusion
Reactive chemical testing is a cornerstone of chemical process safety and industrial risk management. By identifying thermal stability, compatibility issues, and runaway reaction potential, organizations can design safer processes, avoid costly incidents, and ensure compliance with safety regulations.
Investing in early and thorough reactivity testing improves operational safety, supports regulatory filings, and safeguards personnel and the environment. As chemical processes become more complex, proactive risk assessment using validated testing methods becomes not just beneficial—but essential.