Introduction
Reactive chemical testing in the pharmaceutical industry plays a crucial role in ensuring both process safety and product integrity. With modern drug development involving increasingly complex chemical reactions, the risk of thermal instability, runaway reactions, and material incompatibility grows significantly—especially during scale-up. Identifying these hazards early through targeted testing is vital for safe, compliant, and efficient pharmaceutical manufacturing.
Why Reactive Chemical Testing Matters in Pharma
In pharmaceutical operations, chemical reactions must be tightly controlled. As processes move from the lab to commercial scale, unexpected hazards can emerge—leading to dangerous outcomes such as fires, explosions, or product degradation.
Reactive chemical testing helps pharmaceutical manufacturers:
-
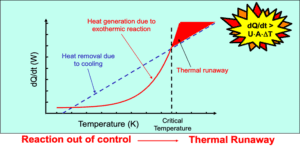
Prevent thermal runaway during scale-up
-
Meet regulatory requirements like OSHA, PSM and ICH Q9
-
Ensure API and excipient compatibility
-
Preserve product stability and quality
Without a solid understanding of the reactive behavior of materials, critical process decisions may be based on assumptions—putting safety, compliance, and patient outcomes at risk.
Common Reactive Hazards in the Pharmaceutical Industry
Pharmaceutical processes face unique reactive risks, such as:
-
Thermal decomposition of APIs and excipients (e.g., peptides, corticosteroids)
-
Runaway reactions in high-energy chemistries (e.g., Grignard, nitration)
-
Peroxide formation in ethers like THF and diethyl ether
-
Moisture-induced incompatibilities during granulation or lyophilization
Recognizing and managing these hazards early is key to successful scale-up and regulatory approval.
Core Methods for Reactive Chemical Testing
Here are the most common testing methods used in the pharmaceutical industry:
1. Differential Scanning Calorimetry (DSC)
DSC detects exothermic decomposition, providing onset temperatures and heat release values. It’s essential for screening raw materials, intermediates, and formulations.
2. Reaction Calorimetry (RC1)
Used during process development, RC1 calorimetry monitors real-time heat, pH, and gas evolution. It helps quantify energy release and cooling demands in reactions like sulfonation or oxidation.
3. Accelerating Rate Calorimetry (ARC)
ARC evaluates adiabatic behavior and supports emergency relief system design by predicting worst-case runaway scenarios.
4. Thermogravimetric Analysis (TGA)
TGA measures weight loss from decomposition or solvent evaporation, aiding in the characterization of hydrates and degradation kinetics.
5. Compatibility Testing (USP <1059>, ICH Q1A)
Used to detect potential reactions between APIs and excipients or packaging materials through stress testing, HPLC, and impurity profiling.
Integrating Testing with Process Development
To maximize value, reactive chemical data should feed into broader process safety and quality frameworks:
-
Quality by Design (QbD): Defines design spaces that avoid hazardous conditions
-
Process Analytical Technology (PAT): Enables real-time control of critical variables
-
HAZOP studies: Identify risks in steps involving heat, gas, or unstable intermediates
-
Design of Experiments (DOE): Clarifies how temperature, solvent, or catalysts affect both yield and safety
This integration ensures a holistic, science-based approach to pharmaceutical manufacturing.
Regulatory Expectations
Global regulatory agencies expect chemical hazard data as part of drug development and process validation:
-
FDA Process Validation Guidance (2011): Emphasizes process understanding and control
-
ICH Q11: Encourages early identification of reactive risks during drug substance development
-
OSHA PSM: Requires analysis of chemical reactivity hazards for high-risk operations
-
GHS SDS Requirements: Mandate disclosure of thermal stability and reactivity in Section 10
Complying with these regulations requires well-documented and scientifically supported testing data.
Best Practices for Reactive Testing in Pharma
To ensure safe and compliant manufacturing, pharmaceutical companies should:
-
Perform DSC screening early in development for all materials
-
Use reaction calorimetry at pilot scale to uncover hidden hazards
-
Promote collaboration between R&D, engineering, EHS, and QA teams
-
Archive all thermal and chemical data for lifecycle management and regulatory audits
-
Include reactivity profiles in development reports and safety assessments
Case Example: Nitration Scale-Up
During scale-up, a pharmaceutical firm working on a nitration reaction for an API discovered a large exotherm at 95°C via DSC. Subsequently, follow-up RC1 testing revealed insufficient cooling capacity, which posed a serious risk. To address the issue, the team implemented dynamic dosing and improved mixing in a jacketed reactor. As a result, they successfully controlled the reaction and achieved safe commercial production under cGMP.
Conclusion
Reactive chemical testing in the pharmaceutical industry is essential for developing safe, stable, and compliant manufacturing processes. By proactively understanding the thermal and reactive behavior of materials from the earliest stages of development, companies can effectively prevent accidents, efficiently streamline scale-up, and consistentlymeet global regulatory expectations. Moreover, integrating reactive testing with QbD, PAT, and risk management frameworks helps transform chemical insight into operational excellence.