Why Reactive Hazards Are Often Overlooked
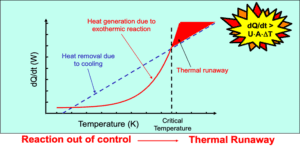
Not all hazards in chemical processing are immediately visible—or even predictable. Reactive chemical hazards often stay hidden until a specific trigger sets off a serious incident. Unlike flammability or toxicity, which are more familiar and easier to quantify, reactivity depends on subtle factors such as temperature, pressure, or material incompatibility.
These incidents often begin with small missteps: a misjudged stability limit, an overlooked incompatibility, or a lapse during storage or scale-up. What starts as a minor oversight can quickly escalate—leading to runaway reactions, pressure buildup, or even explosions. Common triggers include:
- Thermal runaway from self-heating or delayed decomposition
- Incompatible mixing of materials during transfer or cleaning
- Contamination from unintended residues or impurities
- Improper storage conditions like heat, light, or sealed containers
- Aggressive scale-up without proper reactivity testing
Misunderstanding or overlooking these factors can lead to serious consequences. Runaway reactions, over-pressurization, and vessel rupture often don’t result from operator error—they happen because someone didn’t fully understand how the materials respond to thermal stress or mixing conditions.
Testing for Reactivity
Identifying reactive hazards isn’t guesswork—it requires data. Through testing, you can see how materials behave under thermal stress, during storage, or when exposed to potential triggers. The right test method depends on the chemical involved, the specific process, and the type of risk you’re assessing. When applied correctly, these tests define the boundaries of safe operation—before those boundaries are unknowingly crossed.
Key tools include:
Differential Scanning Calorimeter (DSC)
DSC measures heat changes as a material heats or cools, helping detect early signs of decomposition or other thermal activity.
- What it tells you: Onset temperature of decomposition, enthalpy (energy) release, thermal stability
- Application: Early-stage screening, evaluating process intermediates, setting storage and handling limits
Thermogravimetric Analysis (TGA)
TGA tracks weight changes during heating to show decomposition, moisture loss, or volatilization.
- What it tells you: Mass loss rates, thermal decomposition stages, volatility
- Application: Evaluating thermal degradation mechanisms, materials characterization
Accelerating Rate Calorimeter (ARC)
ARC tests how fast a material heats itself and builds pressure under conditions where no heat escapes, simulating worst-case scenarios.
- What it tells you: Self-heating rate, time to runaway, pressure rise
- Application: Process safety evaluations, scale-up assessments, emergency relief sizing
Reaction Calorimetry
This method measures heat released in real-time during a chemical reaction intended reaction conditions.
- What it tells you: Real-time heat release, reaction control parameters
- Application: Optimizing reaction conditions, ensuring safe scale-up, identifying safe process limits
Vent Sizing Package (VSP)
VSP simulates runaway reactions with minimal heat loss to provide data for emergency vent design and pressure relief systems.
- What it tells you: Venting requirements, non-condensable gas generation
- Application: Emergency vent sizing, pressure relief design, thermal hazard assessment
From Test Results to Safer Decisions
Raw data means little unless it’s applied. Reactivity tests must be interpreted in the context of how a material is used—how hot it gets, how long it’s stored, or what it’s mixed with.
For example:
- A DSC result showing decomposition at 110°C isn’t enough—you need to ask: What happens if my process reaches 90°C during a fault? Is that close enough to be dangerous?
- An ARC result showing rapid pressure rise might mean your reactor needs a larger relief valve or better trip logic.
- VSP data can drive the design of your emergency venting system—ensuring it works in worst-case conditions.
The goal is to turn numbers into action: set safe operating ranges, design fail-safes, and prepare for emergencies based on how the chemistry actually behaves—not assumptions.
Building Reactivity into Process Safety
Reactivity analysis shouldn’t sit in a separate binder—it needs to feed directly into your core process safety practices.
When integrated properly, it supports:
- Process Hazard Analysis (PHA):Reactivity data helps identify credible worst-case scenarios.
- LOPA (Layers of Protection Analysis):Guides where safeguards like alarms or relief systems are needed.
- SIS (Safety Instrumented Systems):Enables accurate, chemically informed trip points.
- Relief system design:Ensures vent sizing matches real pressure and gas generation potential.
It also fosters better communication. Chemists, engineers, safety professionals, and operators all need to understand what the test data reveals—and how it informs procedures, equipment design, and emergency planning. As chemical processes grow more complex and global supply chains introduce variability in raw materials and formulations, the chances of encountering unexpected reactivity increase. Whether you’re scaling up production, modifying a formulation, or storing chemicals for extended periods, identifying and managing reactive hazards is essential to prevent catastrophic events.